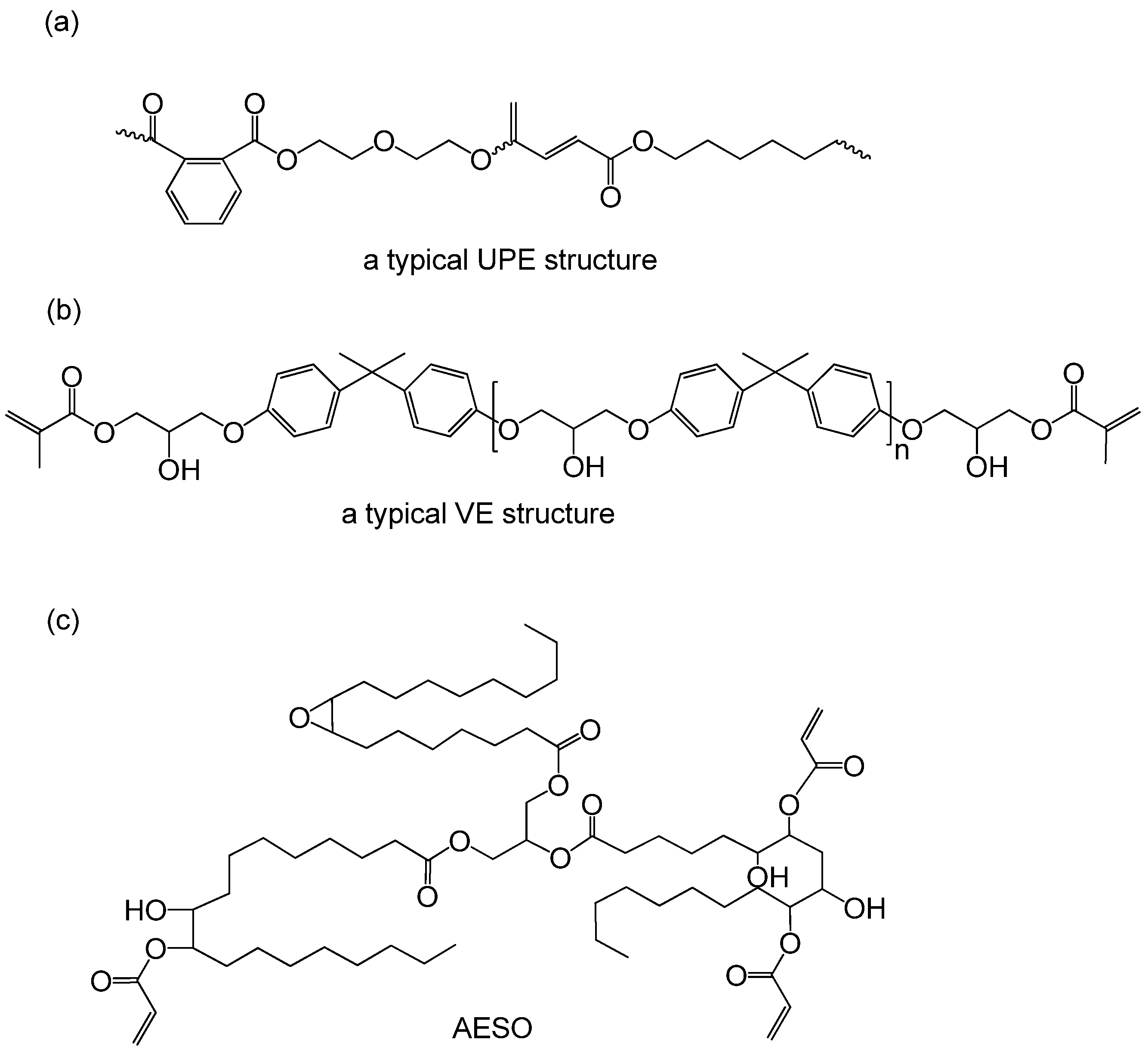
Unsaturated polyester any of a group of thermosetting resins produced by dissolving a low molecular weight unsaturated polyester in a vinyl monomer and then copolymerizing the two to form a hard durable plastic material.
Polyester vinyl monomer.
The resultant polymer can vary from a high viscosity liquid brittle to a low melt solid material.
23 n0 11 pp 921 922 1987 0014 3057 87 3 00 0 00 printed in great britain pergamon journals ltd synthesis of polyester poly vinyl monomer block copolymers by c i simionescu.
The most widely used member of the category is vinyl acetate.
The basic acrylic monomers or oligomers contain unsaturated double bonds vinyl groups and consequently cure by addition polymerization involving a free radical reaction.
It is the ethylene iupac ethene molecule h2c ch2 with one fewer hydrogen atom.
For vinyl adhesives we use a white vinyl monomer.
The material has a thickness of 58 μm and a glossy finish.
In chemistry vinyl or ethenyl 1 abbreviated as vi 2 is the functional group with the formula ch ch2.
E comanita v received 7 july 1987 abstract polycondensations were carried out between succinyl chloride 4 4 azobis 4 cyanovaleryl chloride and bisphenol a to yield copolyesters containing labile n n units.
The name is also used for any compound containing that group namely r ch ch2 where r is any other group of atoms.
The polymer is then dissolved in a liquid reactive vinyl 1 2 ethylenically unsaturated monomer such as styrene vinyl toluene diallyl phthalate or methyl methacrylate to give a solution with a viscosity in the range between 2 0 and 20 0 poise.
Vinyl acetate is not as hydrolytically stable in polymers as are the structurally similar acrylic esters.
Free radical producing compounds such as peroxides.
Vinyl esters undergo homopolymerization via a radical mechanism.
An industrially important example is.
Polymers used in this class of mate rials are the unsaturated polyesters formed by reaction of unsaturated dibasic acids and dihydric alcohols.